
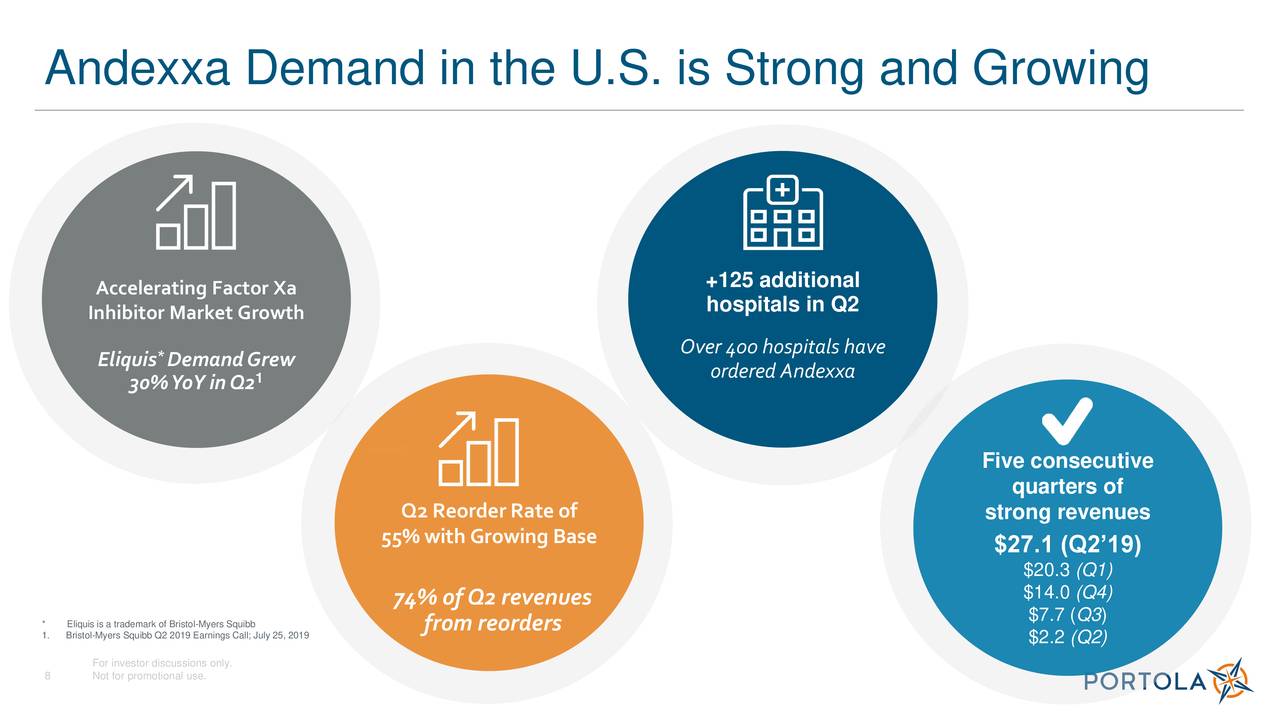
According to EvaluatePharma, Andexxa carries a 2022 sellside revenue forecast of $749m. Portola shares edged up 3% in pre-market trading. Meanwhile, the post-marketing trial is slated to begin in 2019 and be reported in 2023. The company now plans to launch an early supply program of “Generation 1” Andexxa in June, with broader commercial launch anticipated in early 2019, assuming the FDA greenlights the manufacturing process of its Generation 2 product. ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo) is a recombinant modified human factor Xa (FXa) protein indicated for patients treated with rivaroxaban or apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. “We remain committed to our scientific leadership in the fields of thrombosis and hematologic cancers.” “We are proud that Andexxa is a first-in-class medicine discovered in our labs,” said CEO Bill Lis. European regulators gave it a thumbs down. One of them is Portola’s own Bevyxxa, whose approval raised some eyebrows since it barely failed a pivotal trial. In a lengthy press release, Portola did not elaborate on how it resolved the FDA’s previous concerns, choosing instead to highlight the market potential of its reversal agent in an era when Factor Xa inhibitors have become increasing popular. “In other words, at that time, manufacturing had to catch up with clinical.” “Andexxa is a breakthrough product, and as such, clinical was much faster than manufacturing,” they wrote. This is a randomized, multicenter clinical trial designed to determine the efficacy and safety of andexanet alfa compared to usual care in patients presenting with acute intracranial hemorrhage within 6 hours of symptom onset to baseline scan and within 15 hours of taking an oral factor Xa inhibitor. The sponsor address listed is the last reported by the sponsor to OOPD.A spokesperson told me that the CRL Portola received in 2016 “focused almost solely on manufacturing concerns, which Portola addressed.” 121 Seaport Boulevard Boston, Massachusetts 02210 United States
Coagulation factor Xa (recombinant), inactivated-zhzoįor reversing the anticoagulant effect of direct or indirect factor Xa inhibitors in patients experiencing a serious uncontrolled bleeding event or who require urgent or emergent surgeryĪlexion Pharmaceuticals, Inc.


 0 kommentar(er)
0 kommentar(er)
